Although screening assays are critical for identifying new anti-inflammatory compounds, unless these compounds can be formulated into a topical product that delivers the compound across the stratum corneum and down to the target cells in the epidemis and/or dermis, the product will be ineffective. The steps to developing an effective topical product involve: (i) assessing likelihood of skin penetration from molecular weight and log P values, (ii) determining solubility and stability of the anti-inflammatory compound in acceptable formulation solvents, (iii) preparing prototype formulations that are physically stable and which maintain biological activity of the compound, (iv) testing the prototype formulation by Franz cell percutaneous absorption analysis to determine the rate and quantity of compound that can penetrate into human skin, and (v) subjecting the formulation to placebo – controlled clinical studies to determine topical efficacy in a patient population.
The stratum corneum is an effective barrier against entry of foreign objects into the skin, and this includes most proteins, peptides, and even small molecules. Thus, the development of topical products which allow penetration of compounds into the skin is not a trivial undertaking. Typically, unenhanced formulations may “deliver” 0.1% to 1% of a “biologically active” compound across the stratum corneum. Even formulations that are engineered to optimize delivery of a given compound may result in, at best, 10% of the applied dose moving across the stratum corneum and down into the skin. In addition, if the molecule to be delivered into the skin is highly hydrophobic, it will likely pass easily into the stratum corneum but not move into the more aqueous environment of the epidermis (73). Thus, a significant percentage of the active compound in the product will never diffuse through the skin to reach the target cells. To aid in formulation development of a given “active” compound, the use of log P values has become popular to predict efficacy in skin
treated fibroblasts were “merged” and colorized by software analysis to show genes that are upregulated or downregulated (shown here in gray scale) by this compound. (C) In the merged image, arrows point to 2 genes, MCP-1 and MMP-1, that are downregulated in fibroblasts treated with quercetin and one gene, TIMP-1, that is upregulated by this compound.
penetration of a given molecule. Log P measurements show the degree to which a given compound will partition between water and octanol (or other non-miscible solvent). For example, a compound that has a Log P of 1 will prefer an organic solvent to an aqueous one by a factor of 10. A compound with a log P of 0 has an equal affinity for water or an organic solvent. From a topical formulation perspective, compounds that have a logP of around 2.5 will likely have a fairly high probability of skin penetration from a suitable formulation (74). In addition to log P values, the ability of any compound to penetrate the stratum corneum depends on its size. Compounds with molecular weights above 1000 are not going to easily move through the stratum corneum regardless of their log P value. Two other factors that influence skin penetration of any compound from a formulation are the solubility and
Table 2 Effects of Quercetin on Gene Expression in TPA-Treated Human Epidermal Keratinocytes Determined by Integriderm Dermarray™
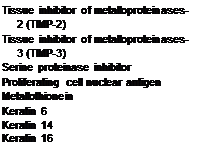 |
 |
Upregulated Downregulated
concentration of the compound in the formulation. Those formulations that contain a nearsaturated (or even super-saturated) concentration of a compound will deliver more of the compound into the skin. Conversely, the more soluble a compound is in the formulation the less potential it will have for leaving the formulation and entering the skin. To increase the movement of compounds into the skin, a number of penetration enhancers may be used. These are solvents that temporarily disrupt the integrity of the stratum corneum allowing molecules to penetrate this layer of skin. Although over 300 penetration enhancers are known, ony a few are used routinely for topical formulation development. Common enhancers used in cosmetic formulations include simple alcohols, propylene glycol, oleic acid, ethoxydiglycol, polyolprepolymer-2 (and PP-14 and PP-15), some terpenoids, cyclodextrins, urea, and sodium lauryl sulfate to name a few (75,76).
