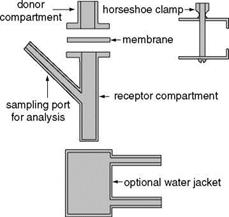
Once a compound’s size, log P value, and solubility properties in various acceptable formulation solvents have been determined, the next step in formulation development involves either measuring the penetration through skin of the compound dissolved in a single solvent or its skin penetration from simple formulations. Regardless of which approach is taken, measuring a compound’s “flux” through skin requires the use of some type of diffusion cell. The most common apparatus for measuring the penetration of topical formulations through skin is the Franz diffusion cell, shown in Figure 11. The unit consists of an upper chamber into which the test formulation is applied and the lower reservoir chamber which is filled with buffer. A piece of human skin (animal skin or a synthetic membrane is sometimes used) is mounted in between the two chambers and held in place with a clamp. The formulation to be tested is applied to the stratum corneum surface of the mounted skin and at various times, samples of the lower reservoir buffer are removed and assayed for the presence of the anti-inflammatory compound in the
formulation. For compounds that are not made radioactive, the presence of the compound in the lower chamber of the Franz cell is typically determined by high-performance liquid dramatography (HPLC) analysis. In order to obtain results which more accurately reflect the rate of skin penetration that will be obtained in vivo, human skin, either dermatomed or full thickness, should be used. The use of Franz diffusion cell analysis to measure percutaneous absorption of anti-inflammatory compounds from topical formulations provides information needed to optimize the formulation prior to initiating clinical studies. Based on dose-response studies in cell culture systems, it is possible to predict what level of skin penetration a given anti-inflammatory compound likely needs to attain to show efficacy in vivo. Formulations can be modified and re-tested by Franz cell analysis until the predicted “flux rate” of compound into the skin is achieved.
It is useful to keep in mind that, as a general rule, compounds which show efficacy in blocking inflammatory mediators in cell culture systems with IC50 values of less than 100 uM (preferably 10 uM) have a reasonable chance of being efficacious when applied topically, assuming the flux rate of the compound from the formulation is optimized. However, topical formulations containing anti-inflammatory compounds that are only effective in cell culture at concentrations higher than 100 uM have a low probability of being good anti-inflammatory products because of the difficulty of delivering enough of the compound into the skin and to the target cells over a long enough period of time to be effective. In our laboratory, compounds with anti-inflammatory IC50 values higher than 100 uM are not considered for product development.
Another consideration when developing topical formulations concerns “residence time” of the active ingredient. To effectively treat inflammatory conditions, a topical formulation must not only deliver enough of the active ingredient into the skin to be effective, but should also deliver the active continuously over many hours. Typically a topical product is applied to the affected area twice a day, once in the morning and once in the evening. Thus, the time between applications can be as much as 12 hours. If the topical formulation delivers a high level of the anti-inflammatory compound into the skin for a short period of time, for example one hour, the compound is going to reach the target cells at a high enough concentration to begin to inhibit inflammation, but after an hour the concentration falls as the active continues to traverse the skin and dissipate into the capillary beds. When one considers that only about 1-2 ml of any topical product is
|
Figure 11 Diagram of Franz diffusion chamber. |
applied to 100 cm2 of skin, developing formulations that deliver enough of the active into the skin continuously over a 12-hour period is not a trivial undertaking. Obviously, if the bioactive compound is effective at nanomolar levels, the product can be designed as an “unenhanced” formulation, which will result in a slower rate of skin penetration and theorectically provide a longer residence time or “depot” of drug needed to affect cell functioning for a 12-hour period. Further, if the water solubility of the active compound is low, it is likely to be retained in the stratum corneum, and only move into the edidermis slowly at a low concentration. If the compound’s bioactivity is in the nanomolar range this low rate of movement into the epidermis will be ideal for maintaining a high residence time in the skin.