Like vitamin C, vitamin E is an essential nutrient, not synthesized by humans and supplied only by oral intake. The main natural sources are fresh vegetables, vegetable oils, cereals,
|
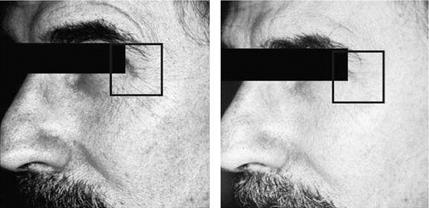
Figure 1 Decrease in small periorbital rhytides after daily application of vitamin C serum 15% (SkinCeuticals) for one year. Source: Photo courtesy of SkinCeuticals, Dallas, Texas, U. S.A. |
and nuts. Natural vitamin E is the most important lipid-soluble, membrane-bound antioxidant in the body. Vitamin E is especially abundant in stratum corneum, delivered there by sebum (17,18). Its concentration is highest at the lower levels of the stratum corneum with a decreasing gradient outward. As the outermost defense of the body, the stratum corneum is first to absorb the oxidative stress of sunlight and pollution. Vitamin E is depleted in the process, so topical application can be particularly advantageous, especially since the lipophilic structure makes it cosmetically attractive for application and absorption.
The redox and free radical chemistry of vitamin E are well-documented (19). The major antioxidant role is the arrest of chain propagation by scavenging lipid peroxyl radicals. One molecule of tocopherol has the ability to scavenge two peroxyl radical molecules (20).
|
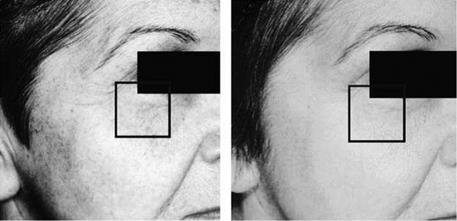
Figure 2 Lightening of UV-induced lentigines and hyperpigmentation after daily application of vitamin C serum 15% (SkinCeuticals) for one year. Source: Photo courtesy of SkinCeuticals, Dallas, Texas, U. S.A. |
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
NADP*
NADPH
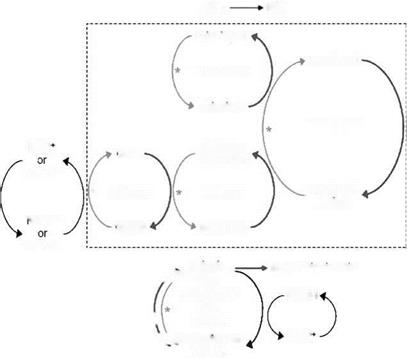
Figure 3 Interactions of low molecular weight antioxidants. The reactions which directly quench oxygen free radicals (RO») are indicated by the dark gray arrows (RO»^RO); the reactions regenerating these antioxidants are also indicated by the light gray arrows. Reactions with arrows touching are directly linked. RO» generated in a cell membrane is reduced by tocopherol, forming a tocopheryl free radical which can in turn be quenched within the membrane by ubiquinol or at the membrane-cytosol junction by ascorbate (vitamin C). RO» generated in cytosol is directly reduced by ascorbate. The oxidized dehydroascorbate is reconverted to ascorbate by glutathione (GSH). Both a-lipoic acid and dihydrolipoic acid (DHLA) directly reduce oxygen free radicals. Also DHLA is itself a potent reducing agent which regenerates the oxidized forms of vitamin C, vitamin E, and oxidized glutathione (GSSH); this linkage is indicated by an asterix. Source: Adapted from Refs. 21, 22.
As shown in Figure 3, several hydrophilic coantioxidants, such as ascorbate and glutathione, regenerate vitamin E from the tocopheryl radical and thereby enhance the antioxidant capacity of vitamin E (21-23). Also, ubiquinol (coenzyme Q10) protects a-tocopherol from photo-oxidation by recycling (24).
There is extensive scientific evidence from animal studies that vitamin E is photoprotective. Topical vitamin E [even the metabolically less potent racemic or ester forms (see “Challenges in Formulation” below)] significantly reduces acute erythema, edema, and sunburn (25-30) if applied prior to UV exposure or (in some studies) immediately after. This has been confirmed histologically by decreased “sunburn cells” (27) and by electron microscopy showing epidermal cell repair and anti-inflammatory effects (28). Less DNA photodamage after UV with concomitant decreased p53 expression has been observed (29). Topical all-rac-a-tocopheryl acetate applied before UV exposure protected the hairless mouse epidermis against decreased DNA-thymidine incorporation and lipid peroxidation; given orally, this protected only against lipid peroxidation (30). This protection results from antioxidant (31) and/or anti-inflammatory activity (32,33).
UV radiation directly alters DNA and induces free redicals (34,35) and epidermal lipid peroxidation (36), thereby initiating and promoting skin cancer (37). Vitamin E protects the skin from this chronic damage by: (i) quenching free radicals [as confirmed in vitro by protection by reducing radiation-induced lipid peroxidation (38)], and (ii) protecting specific membrane proteins containing Se or sulfur (39). Indeed, all-roc-a – tocopherol has been shown to prevent epidermal chemical carcinogenesis (40-42) as well as UV-induced photocarcinogenesis (43-46).
In hairless mice both oral (43) and topical all-roc-a-tocopherol combined with ascorbic acid (44) increased the latency period and decreased the number of UV-induced tumors. In Skh:2 hairless mice, both topical d-a-tocopherol (5%) and d-a-tocopheryl succinate (5%) as well as oral d-a-tocopheryl acetate signifcantly retarded the onset and decreased the incidence of UV-induced skin tumors; the topical succinate was less effective than the other two forms (25).