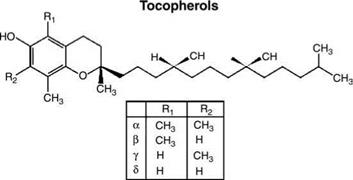
Several forms of vitamin E exist in natural dietary sources. The form which is found in mammalian tissues and has by far the greatest biologic activity is pure, nonesterified d-a-RRR-tocopherol (47,48) which has three methyl groups on the 6-chromal ring (Fig. 4). Humans use predominantly a-tocopherol because a specific a-tocopherol transfer protein selectively transfers a-tocopherol into lipoproteins (49). The other natural forms are beta, gamma, and delta which contain only one or two methyl groups on the 6-chromal ring. Relative to the alpha form, the beta, gamma, and delta RRR-tocopherols give only 42%, 72%, and 40%, respectively, of the protection against post-UV edema (50). The synthetic form is “dl” or “all-roc,” a mixture of eight stereoisomers. Not only is the decreased activity of the all-roc mixture of vitamin E important (51), but also the mixed all – roc form of vitamin E has been reported to cause allergic contact dermatitis (52) and erythema multiforme (53) when applied topically. No such adverse reactions have been reported with pure d-a-tocopherol.
Instead of the pure d-a-tocopherol, the synthetic isomers are esterified (to acetates and succinates) for use in commercial vitamins and some topical formulations because the esters are far more stable. The ester vitamin E acetate has been shown to be absorbed into the skin (54-56). This ester must be hydrolyzed to the active free tocopherol form before there is any biologic activity, a reaction which readily occurs in the stomach after oral ingestion or in cell and organ culture, but there is conflicting evidence as to what extent
|
Figure 4 Molecular structures of tocopherols. |
this conversion occurs, especially in the stratum corneum (57-59). Thus the antioxidant potential of esterified vitamin E is far less than the natural tocopherol form (60). There is greater bioconversion in the lower nucleated epidermal cells (58,59) depending on the formulation (61). UVB exposure may enhance this conversion (62).
Stabilization of the non-esterified d-a-tocopherol to give a product an effective long shelf-life is a challenge in formulation. The stability can be enhanced by packaging in dark, sealed ampules for one application-only delivery, by formulating within liposomes, or by stabilizing chemically, often using other antioxidants. (Patents are pending for the latter two methods).