Personal cleansing products are complex systems that often contain several surfactants. Even a seemingly simple cleanser such as a soap bar comprises a mixture of soap species. Several of the mechanisms believed to drive surfactant interactions with the skin are discussed below. These are presented separately for convenience but the mechanisms are undoubtedly interdependent to some degree.
Surfactant Structural Considerations
The surfactant composition of a personal cleanser in large part determines the product’s potential to impact skin, and there are numerous published studies that compare and
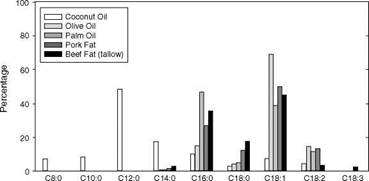
contrast the skin effects of individual surfactants and full formula cleansers. But skin compatibility can vary even within a given surfactant type. Soap provides a good example. As defined previously, soap is the alkali salt of a fatty acid. The regulatory definition of soap is quite narrow and only a few true soaps remain on the market in the United States (35-37), but products based on soap-syndet mixtures (so-called combo bars) persist in the U. S. market, and soap remains a popular cleansing form in many other countries. The raw material used in soap manufacture is often a mixture derived from tallow, vegetable oils, and their processed derivatives (38,39). Being derived from natural sources, these raw materials comprise a mixture of fatty acid species. The fatty acid compositions of triglycerides from several different sources that are used in soap manufacture are shown in Figure 4 (21).
The varying composition of the raw materials used in soap manufacture means that saponfication, i. e., reacting triglyceride with alkali to form soap and glycerin, yields a mixture of soap species. The chemical composition of the finished soap product determines its skin compatibility. For example, Dahlgren et al. used soap bars prepared with different relative amounts of tallowate and cocoate soaps to demonstrate that the level of dryness and erythema following controlled washing is dependent on the ratio of these soap species (40). In this work a bar based entirely on coconut-derived soap was harshest to skin, while a bar based entirely on tallow-derived soap was mildest. The mildness of bars based on intermediate combinations of cocoate and tallowate soap fell between these extremes. These results, and the differences in the fatty acid compositions of the raw materials (Fig. 4), indicate that the distribution of soap species in a personal cleansing product is an important determinant of its skin compatibility.
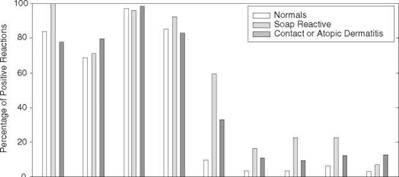
Studies conducted with pure fatty acids demonstrate this effect at a more fundamental level. Blank conducted patch tests using coconut oil and pure fatty acids commonly found in soap (41). Patches were applied for 24 hours to intact skin on the upper arms of normal (healthy) subjects, subjects who previously exhibited a reaction to soap (pruritus or vessiculation), and subjects with evidence of contact or atopic dermatitis. Reactions were read one hour after patch removal. The results are summarized in Fig. 5. The percentage of positive reactions in each test group shows clear fatty acid chain length dependence, with the incidence decreasing as the chain length increases. Kellum
|
Fatty Acid Compostion of Triglycerides from Various Sources
(Caprilic) (Capric) (Lauric) (Myristic) (Palmific) (Stearic) (Oleic) (Linoleic) (Linolenic) Fatty Acid Species (carbons:double bonds) Figure 4 Typical fatty acid composition of some triglycerides commonly used to manufacture soap. Source: From Ref. 21. |
|
conducted a similar study, patching saturated fatty acids with even-numbered chain lengths from C2-C16 on the backs of healthy volunteers for up to 15 days (42). Response was greatest to the C8-C12 acids, with the C12 homologue producing the most severe reactions. The author hypothesized that the C12 chain length might be optimum for incorporation into or passage through biological membranes. Stillman et al. conducted patch testing with even – and odd-numbered saturated fatty acids ranging from C3-C18; several unsaturated C18 species were also tested (43). The results again showed the greatest reaction to the C8-C12 acids. Of the unsaturated species tested, only linoleic acid (C18:2) produced irritation.
Garcia-Dominguez and coworkers proposed a five-step model to account for the increased irritancy of C12 ionic surfactants (44). The model involves both ionic and hydrophobic interaction between the surfactant molecule and proteins at the skin surface, ultimately leading to migration of the charged and hydrophobic portions of the surfactant molecule into the protein. Irritation results from localized environmental changes within the protein structure induced by the presence of surfactant. Thus, the higher irritancy of C12 surfactants is again attributed to structural characteristics that favor their interaction with the skin.
These studies show that soap composition, in particular the chain length distribution of fatty acids in the soap, is an important determinant of soaps’ skin compatibility. Using tailored mixtures of fatty acid starting material, in which the longer chain length species predominate, is one approach that is used to effectively improve the skin compatibility of soap bars (45,46).
The skin compatibility of many synthetic detergents exhibits a structural dependence similar to that of soap. Kligman and Wooding used patch testing to estimate the ID50, the concentration needed to produce a discernible irritant reaction in 50% of the study population in 24 hours, and the IT50, the number of days of continuous exposure to produce a threshold reaction in 50% of the study population, for a series of sodium alkyl sulfates (47). They observed minimum values for both parameters, i. e., greatest irritancy, for the C12 chain length (SLS). Dugard and Scheuplein measured the effect of C8-C16 homologues of the sodium salts of primary aliphatic acids (soap), sodium n-alkyl sulphates, and n-alkylamine hydrochlorides on the permeability of human epidermal
membranes (48). They observed the greatest permeability increase with the C12 and C14 members in each series. Robbins and Fernee reported a maximum in the swelling behavior of epidermal membrane, a parameter reported to parallel anionic surfactants’ ability to elicit erythema in vivo, for the C12 homologue in a series of alkyl sulfates (49). They note that surfactant binding to keratin is also optimal at this chain length. Rhein et al. used a similar experimental procedure and reported maximal swelling for the Ci2 or C14 homologues of alpha olefin sulfonates, paraffin sulfonates, linear alkylbenzene sulfonates, and alkyl sulfates (23). Increases in swelling response with time suggested surfactant effects on keratin secondary and tertiary structure. Imokawa and co-workers conducted experiments using a surfactant solution circulation apparatus to assess the skin roughening potential of C8-C14 soaps and of homologous series of various synthetic surfactants (50,51). The C12 soap and synthetic surfactants produced the greatest skin roughening effect within each homologous series.
These examples illustrate a common surfactant feature that reduces skin compatibility, namely, a chain length of about C12. Thus, one way to improve skin compatibility of syndet-based cleansers is to minimize their content of short-chained surfactants, especially C12 species, analogous to the soap bar example given earlier. Using a modified surfactant can also improve skin compatibility. For example, Rhein et al. reported a reduction in stratum corneum swelling produced by C12-C14 alkyl ethoxy sulfates as the degree of ethoxylation increases (23). Finally, personal cleansers’ skin compatibility is often improved by using mixtures of different synthetic surfactants (23,24,28,52).