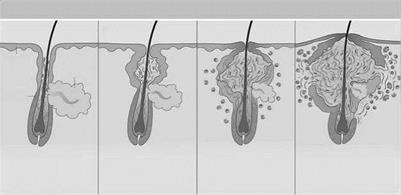
Follicular epidermal hyperproliferation results in the formation of the primary lesion of acne, the microcomedo. The epithelium of the upper hair follicle, the infundibulum, becomes hyperkeratotic with increased cohesion of the keratinocytes. The excess cells and their tackiness result in a plug in the follicular ostium. This plug then causes downstream concretions of keratin, sebum, and bacteria to accumulate in the follicle. These packed concretions cause dilation of the upper hair follicle producing a microcomedo. The stimulus for keratinocyte hyperproliferation and increased adhesion is unknown. However,
|
|||
|
|||
|
|
||
|
|||
|
|||
|
![]()
Epithelium
Figure 1 Acto Pathogenesis. The four key steps in acne pathogenesis: (A) follicular epidermal hyperproliferation, (B) excess sebum production, (C) inflammation, and (D) the presence and activity of Propionibacterium acnes. Source: Adapted from Ref. 11.
several proposed factors in keratinocyte hyperproliferation include: androgen stimulation, decreased linoleic acid, and increased interleukin-1 alpha (IL-1 alpha) activity.
Androgenic hormones may act on the follicular keratinocytes stimulating hyperproliferation. Dihydrotestosterone (DHT) is a potent androgen that may play a role in acne. Figure 2 demonstrates the physiologic pathway for dehydroepiandrosterone sulfate (DHEA-S) conversion to the androgen DHT. 17-beta HSD and 5-alpha reductase, are enzymes responsible for converting DHEA-S to DHT. When compared to epidermal keratinocytes, follicular keratinocytes have increased 17-beta HSD, and 5-alpha reductase thus enhancing DHT production (12,13). DHT may stimulate follicular keratinocyte proliferation. Also supporting the role of androgens in acne pathogenesis is the evidence that the individuals with complete androgen insensitivity do not get acne (14).
Follicular keratinocyte proliferation may also be regulated by linoleic acid. Linoleic acid is an essential fatty acid in the skin that is decreased in subjects with acne. The quantity of linoleic acid normalizes after successful treatment with isotretinoin. Subnormal levels of linoleic acid may induce follicular keratinocyte hyperproliferation, and produce proinflammatory cytokines. It has also been suggested that regular quantities of linoleic acid are actually produced but are simply diluted by increased sebum production (15).
In addition to androgens and linoleic acid, IL-1 may also contribute to keratinocyte hyperproliferation. Human follicular keratinocytes demonstrate hyperproliferation and microcodemo formation when IL-1 is added. IL-1 receptor antagonists inhibit
|
3-beta-HSD |
|
|
DHEA —————— |
androstenedione |
|
^aromatase |
17-beta-HSD |
|
estrone |
і testosterone |
|
17-beta-HSD |
5-alpha-R |
|
aromatase |
|
|
estradiol |
dihydrotestosterone androstanediols |
|
3-beta and 3-alpha-HSDs |
Figure 2 Steroid metabolic pathway. DHEA is a weak androgen that is converted to the more potent testosterone by 3-beta-HSD and 17-beta-HSD. Five-alpha-reductase then converts testosterone to dihydrotestosterone, the predominant hormonal effector on the sebaceous gland. Both DHEA and testosterone can be metabolized to estrogens by the enzyme aromatase. The sebaceous gland expresses each of these enzymes. Source: Adapted from Ref. 11.
microcodemo formation providing additional support for the cytokine’s role in acne pathogenesis (16,17).

