Although careful and thorough analysis of the biological activities of a given antiinflammatory compound using a variety of cell culture models can provide information on which inflammatory conditions a given compound is likely to be effective in treating, and although skin penetration studies will aid in the development of a formulation that theoretically delivers adequate levels of the compound into the skin, of course the only way to know if the topical formulation is truly effective in treating inflammatory conditions is to conduct clinical studies. In this regard, there are several different approaches to designing and implementing a clinical study. The least scientifically credible study design is one in which no placebo is run, where there is no blinding of either the clinical investigator or patients, and where the efficacy of a product formulation is simply determined comparing some parameter (redness, tone, skin roughness, etc.) at the end of the treatment period to baseline readings determined at the beginning of the study. In order to determine the efficacy of a novel anti-inflammatory compound in a formulation, it is necessary to conduct clinical studies under blinded, placebo-controlled conditions, where the efficacy of the formulation containing the anti-inflammatory “active” is statistically compared to the placebo group.
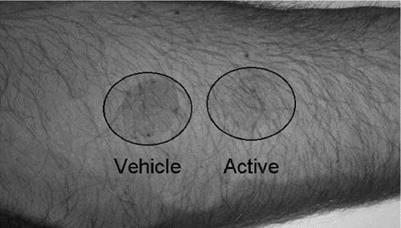
One of the easiest and quickest clinical studies to conduct to assess the potential antiinflammatory activity of a topical formulation is a UVR erythema study. In this protocol, the patient is exposed to a 3 MED dose of UVB radiation from a light source that irradiates a small area (20 mm diameter) of skin. Multiple areas on the inner arm are irradiated. Immediately after irradiation, one spot is left untreated while a second spot is treated with the topical formulation containing the anti-inflammatory compound. The third irradiated area is treated with a “vehicle” lotion that is identical to the treatment lotion but does not contain the putative anti-inflammatory compound. For these studies it is important that the skin is not pre-treated with the test lotions. The reason for this is that if the putative antiinflammatory compound in the formulation absorbs UV light, then applying the product before irradiation may result in protection from erythema simply because of the UV absorbing properties of the compound. At hourly intervals after irradiation, surface spectrophotometric measurements and photographs of the treated areas are taken to quantify the level of erythema. Clinical photographs of one patient from a study conducted on a novel anti-inflammatory compound developed in our laboratory are shown in Figure 12. The photograph shows that even 24 hours after irradiation and after a single application of an anti-inflammatory formulation, the area treated with this formulation has markedly less erythema than the area treated with the vehicle formulation (the exact formulation but without the anti-inflammatory compound).
|
Figure 12 Effect of anti-inflammatory topical formulation on UVB radiation-induced sunburn 24 hours post-irradiation. |
By using multiple cell culture-based inflammatory mediator assays to identify the antiinflammatory capabilities of a given compound, followed by the development of topical formulations that are analyzed by Franz cell percutaneous absorption analysis to ensure that adequate amounts of the compound are being delivered into the skin, it is possible to develop novel topical anti-inflammatory products that have a very high probability of being effective treatments for a variety of inflammatory skin conditions. There are a number of botanically derived compounds which have been shown to have excellent antiinflammatory activity, and results of screening assays in our laboratory suggest that perhaps as many as 50 fairly common botanically derived compounds could be developed into topical anti-inflammatory products that would effectively lower the level of many inflammatory cytokines and chemokines in the skin including PGE-2, IL-1, TNF-alpha, MCP-1, IL-12, and IL-8. Further, these compounds can not only block the production of cytokines but can also suppress the ability of a target cell to respond to a given cytokine or chemokine. When one considers the known deleterious side effects that have been reported for the anti-inflammatory steroids and recently for the newer class of oral or injectable anti-inflammatory immunomodulator drugs, it seems that the development of topical antiinflammatory products that are less immunosuppressive and which are delivered directly to the affected areas of the skin instead of systemically might represent a safer approach. Such products could be designed to reduce or “reset” cytokine and chemokine levels in affected areas of the skin to a more non-inflamed “ground state.” Such a product would reduce the inflammatory response but yet leave the immune system intact to fight infection and to conduct surveillance. From our research it appears very likely that a number of botanically based compounds could be formulated into topical products to meet this goal.