Absorption is only one way by which light can interact with the skin. Absorption of the UV and visible light in skin is due to electronic excitation of aromatic or conjugated unsaturated chromophores. A chromophore is a chemical that absorbs light with a characteristic spectral pattern. There are many kinds of chromophores in the skin, but a few major chro – mophores predominantly determine the optical absorption within each skin layer [6,7]. Spectral ranges of absorption of the main skin chromophores are presented in Fig. 3.3.
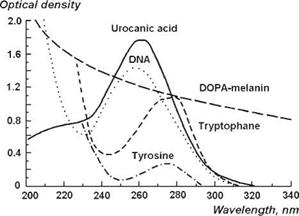
Proteins found in the epidermis contain the aromatic amino acids tryptophan and tyrosine which have a characteristic absorption band near 270-280 nm; urocanic acid and the nucleic acids also contribute to this absorption band with a maximum near 260-270 nm (Fig. 3.4). Epidermal melanin plays an important role in limiting the penetration depth of light in the skin [8]: it effectively absorbs at all wavelengths from 300 to 1200 nm, but the strongest absorption occurs at shorter wavelengths, in the near-UV spectral range (Figs. 3.4 and 3.5).
In the IR spectral range, the skin absorption spectrum is essentially determined by the absorption of water in the skin (Fig. 3.2).
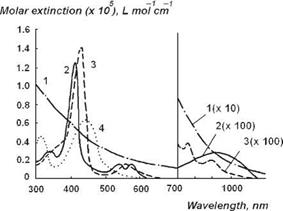
Some of the major dermal chromophores are oxyhemoglobin, deoxyhemoglobin, bilirubin, carotenoids, and porphyrins (Fig. 3.5). Both the oxygenated and deoxygenated forms of hemoglobin absorb light. Oxyhemoglobin has its strongest absorption band at 415 nm (Soret band), and it has two secondary absorption bands at 542 and 577 nm (Q bands). Deoxyhemoglobin has primary absorption band at 430 nm and it has a single secondary absorption band at 555 nm. Both hemoglobins exhibit the lowest absorption at wavelengths longer than 620 nm. Bilirubin has two relatively broad absorption bands near 330 and 460 nm.
The absorption of diffuse light by skin pigments is a measure of bilirubin content, hemoglobin concentration and its saturation with oxygen, and the concentration of pharmaceutical products in blood and tissues; these characteristics are widely used in the diagnosis of various diseases (Fig. 3.5). Certain phototherapeutic and diagnostic modalities take advantage in dependence of transdermal penetration of visible and near infrared (NIR)
Carotenoids —————-
Porphyrins*
Bilirubin*
FAD* ——– ————– ————————-
NADH* ——– —————–
NAD* ——–
Tyrosin* —
Tryptophane* __ _______
DNA ————-
Urocanic Acid* ————-
Fatty Acids ————————
Proteins ——————————
Hemoglobin ——————————————————-
Melanin
і——- 1——- 1————– 1—- 1——— 1———- 1—- >——– 1———- ‘——- 1———— 1——– 1
200 250 300 350 400 450 500
Wavelength, nm
Figure 3.3 Spectral ranges of absorption of the main skin chromophores. For chromophores marked with (*), range indicated is a half-width of the band [7].
|
Figure 3.4 UV absorption spectra of major chromophores of human skin [DOPA-melanin, 1.5 mg % in H2O; urocanic acid, 104 M in H2O; DNA, calf thymus, 10 mg % in H2O (pH 4.5); tryptophan, 2 x 104 M (pH 7); tyrosine, 2 x 104 M (pH 7)] [6]. |
|
Figure 3.5 Molar attenuation spectra for solutions of major visible light-absorbing human skin pigments: 1, DOPA-melanin (H2O); 2, oxyhemoglobin (H2O); 3, hemoglobin (H2O); 4, bilirubin (CHCl3) [6]. |
light inside the body in the wavelength region corresponding to the therapeutic or diagnostic window (600-900 nm) (Fig. 3.2).
Human skin contains various types of native fluorophores (chromophores that emit light with a characteristic spectral pattern) at its excitation by a proper wavelength with unique absorption and emission spectra, different fluorescence quantum efficiency, different fluorescence decay time, and different distribution within the skin. Some fluorophores have similar absorption and fluorescence spectra, and typically, fluorescence spectra measured on the skin surface are the result of the overlapping bands of various such fluorophores. The skin also contains nonfluorescent chromophores, such as hemoglobin and melanin. These chromophores may absorb fluorescence light emitted by the other fluorophores, and thus may modify initial fluorescence spectra due to filtering properties of nonfluorescing chromophores.
When the excitation wavelength is increased, new fluorophores are involved in the formation of the shape of fluorescence spectrum. The closer the excitation wavelength to the center of the so-called therapeutic/diagnostic window (600-900 nm), the larger the penetration depth of the excitation light in tissue, and the larger is the tissue volume probed by the excitation light. As a result, new kinds of fluorophores located in deeper skin layers contribute to the total tissue fluorescence measured.
The spectral ranges of fluorescence of the main skin fluorophores are presented in Fig. 3.6 [7]. It can be seen that the skin autofluorescence (AF) (natural fluorescence of a tissue) in the UVA range is dominated by the fluorescence bands of aromatic amino acids, namely tyrosine and tryptophan. Tyrosine and tryptophan content in epidermis is more than twice that of the whole skin, and this is why epidermis has high AF in the UVA range. This also explains why the fluorescence of psoriatic stratum corneum is significantly higher than that for normal stratum corneum.
Among the endogenous skin fluorophores are the different forms of nicotinamide adenine dinucleotide (NAD) and keratin located in the epidermis and dermal collagen. The reduced (NADH) and oxidized (NAD+) forms of NAD take part in cellular metabolism, and the intensity of their specific fluorescence (fluorescence maxima near 460 nm and 435 nm, respectively) is used for the quantitative NADH detection and differential diagnostics of the metabolism dysfunction. A similarity between the AF spectrum of the human skin in vivo and the emission spectrum of the keratin (maximum near 450 nm) was found.
Collagen is one of the most important skin fluorophores. Approximately, 75% of the dry weight of the dermal tissue is composed of the collagen fibers. Collagen is the main structural component of the connective tissue and accounts for about 90% of protein in human dermis. Dermis holds a thin fibrillar network mainly composed of Types I (about 80%) and III (about 20%) collagen. Collagen of Type IV is found in the cellular basement membrane,
which is connected to the collagen network by anchoring fibers containing collagen VII [12]. Collagen fibers exhibit a constant density throughout all the dermal layers.
All absorption spectra of different chromophores can be the function of temperature and light intensity. For example, absorption spectrum of whole blood can be dramatically changed in the range from normal body temperature to temperature of water vaporization. It can be explained by methemoglobin formation and coagulation of blood. Another example is shifting of water absorption spectrum to the shorter wavelength range at temperature increase [13] (Fig. 3.7).