The “brick and mortar” model of the SC has been known for many years. However, a more complete description of this model includes “corneodesmosomes.” Corneodesmosomes (26) are macromolecular glycoprotein complexes incorporated into the CE and consist of the cadherin family of transmembrane glycoproteins, desmoglein 1 (Dsg 1) and desmocollin 1 (Dsc 1). These glycoproteins span the cornified envelope into the lipid – enriched intercellular space between the corneocytes and provide cohesion by binding homeophilically with proteins on adjacent cells. Within the corneocytes, Dsg 1 andDsc 1are linked to keratin filaments via corneodesmosomal plaque proteins such as plakoglobin, desmoplakins, and plakophilins. The corneodesmosomal protein, corneodesmosin (Cdsn), after secretion by the lamellar bodies with the intercellular lipids and certain proteases, becomes associated with the desmosomal proteins just before transformation of desmosomes into corneodesmosomes. As these proteins are cross-linked into the complex by transglutaminase, their controlled disruption must occur by proteolysis to allow
|
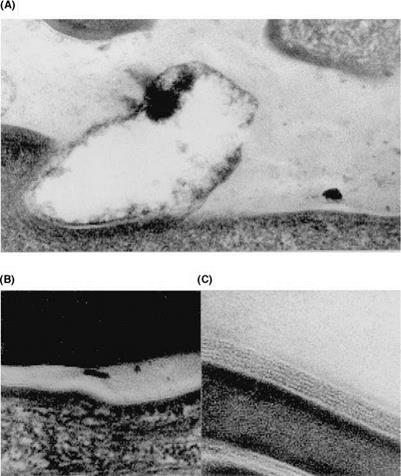
Figure 7 Organization of stratum comeum lipids in tape strippings of individuals with clinically normal skin. Transmission electron micrographs of tape strippings. Ultrastructural changes in lipid organization towards the surface of the stratum corneum: (A) First strip; absence of bilayers and presence of amorphous lipidic material. (B) Second strip; disruption of lipid lamellae. (C) Third strip; normal lipid lamellae. x200,000. Source: From Ref. 23. |
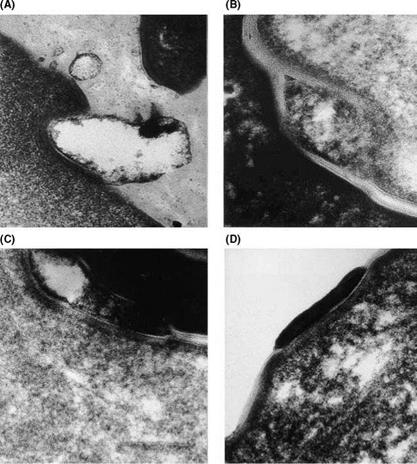
desquamation to proceed. Indeed, Rawlings et al. (Fig. 8) (23) demonstrated degradation of the corneodesmosomes towards the surface of the SC in humans.
Desquamation is facilitated by the action of specific hydrolytic enzymes in the SC that degrade the corneodesmosomal linkages. Currently, several serine, cysteine, and aspartic enzymes are believed to be involved in this process, namely stratum corneum chymotryptic enzyme (SCCE), stratum corneum tryptic enzyme (SCTE), stratum corneum thiol protease (SCTP, now known as Cathepsin L-2), cathepsin E, and the aspartic protease cathepsin D. SCCE and SCTE are alkaline-optimal enzymes whereas the latter ones are acidic-optimum enzymes (27-31). Cathepsin L has also recently been implicated in Cdsn hydrolysis (32). Only SCTE and not SCCE, however, was capable of degrading Dsg 1 (33). This enzyme was also reported to be involved in the processing of pro-SCCE. Bernard et al. (34) have also identified an endoglycosidase, heparanase 1, within the SC, thought to play a role in the preproteolytic processing of the protecting sugar moieties on corneodesmosomal proteins.
|
Figure 8 Electron micrographs of tape strippings of normal skin (grade 1). Degradation of corneodesmosomes (CD) toward the surface of the stratum corneum: (A) First strip; CD fully degraded. (B) Second strip; CD partially degraded and encapsulated by lipid lamellae. (C) Third strip; CD partially degraded, vaculation of structure. (D) Third strip, normal CD in contact with lamellar lipids. Source: From Ref. 23. |
Cdsn undergoes several proteolytic steps. Cleavage of the N terminal glycine loop domain occurs first at the compactum disjunctum interface (48-46 KDa to 36-30 KDa transition), followed by cleavage of the C terminal glycine loop domain in exfoliated corneocytes (36-30KDa to 15KDa transition) (35). The last step appears to be inhibited by calcium resulting in residual intercorneocyte cohesion. Nevertheless, the presence of oligosaccharides did not protect Cdsn against proteolysis by SCCE (33). A complete list of the putuative desquamatory enzymes is given in Table 1.
These enzymes largely exist as proforms, and as they are secreted with the lamellar bodies, they have been immunolocalized to the intercorneocyte lipid lamellae. Sondell et al. (36) used antibodies that immuno-react precisely with pro-SCCE to confirm that this enzyme is transported to the SC extracellular space via lamellar bodies. In later studies, using antibodies to both pro-SCCE and SCCE, Watkinson et al. (37) demonstrated that the processed enzyme was more associated with the corneodesmosomal plaque. More recently, Igarashi et al. (38) have immunolocalized cathepsin D to the intercellular space,
 |
|
Table 1 Desquamatory Enzymes
whereas cathepsin E was localized within the corneocytes. Finally, KLK8 has also been reported to be localized to the intercellular spaces of the SC (39).
As the desquamatory enzymes are present in the intercellular space, the physical properties of the SC lipids, together with the water activity in this microenvironment, will influence the activity of these enzymes. Interestingly, however, SCCE appears to have a greater tolerance to water deprivation than other proteolytic enzymes, and this may be an adaptation to maintain enzyme activity even within the water-depleted SC intercellular space (40). However, a variety of inhibitors are also present to attenuate their activities, cholesterol sulphate being one of them. Other protein and peptide inhibitors are present such as elfin, covalently bound to the CE, antileukoproteinase, alpha-1-antitrypsin, alpha-1- antichymotrypsin, and the SPINK5-derived peptides (41). Nevertheless, anti-leukoprotease is believed to be the major physiological inhibitor of SCCE; the serpins are too low in concentration to be physiologically relevant (42). Caubet et al. (33) recently speculated in a new model of desquamation that SPINK5 may also inhibit SCTE.
Currently, little is understood of the molecular activation mechanisms of SCCE or other enzymes within the SC, but Brattsand et al. (43) has proposed a model recently for the activation of the kallikreins (Fig. 9). Clearly, SC pH and water content will influence enzymic activity. As the SC pH declines towards the surface of the skin, the activity of SCTE and SCCE may be reduced and perhaps the acid optimal cathepsin enzymes mediate the final desquamatory steps. The role of the newly identified skin aspartic protease and caspase 14 in this process is still awaiting clarification.