Salicylic acid (orthohydroxybenzoic acid) (Fig. 5) is an aromatic hydroxyacid with the hydroxyl and carboxyl groups attached directly to a benzene ring (47,48). It is frequently referred to as a beta-hydroxy acid, but a more accurate description is a phenolic aromatic acid because the carboxyl and hydroxyl groups are present on the benzene ring; in this position, the hydroxyl group is acidic (2,47,48). This is to be compared to the hydroxyl group of conventional alpha- or beta – hydroxy acids, which have their attachment on an
|
COOH
|
|
|
Figure 5 Salicylic acid, orthohydroxybenzoic acid.
|
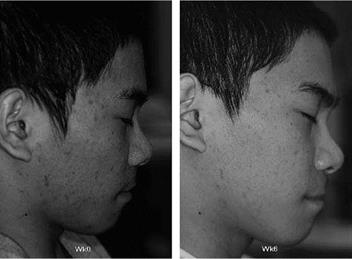
Figure 6 Mild acne: baseline and after six weeks. Twice-daily application of a topical solution containing 2% salicylic acid in combination with 4% alpha-hydroxy acids (benzilic acid, citric acid, tartaric acid, and O-acetylmandelic acid). |
aliphatic or alicyclic structure, rendering the hydroxyl group chemically neutral (48). This chemical difference appears to differentiate the activity of salicylic acid from AHAs on the skin (8).
Salicylic acid has been used as a keratolytic since the 1800s, when it was first derived from the bark of the willow tree (49,50). Its effects on skin have been studied and determined to be primarily limited to enhanced shedding of the stratum corneum, with no increase in mitotic activity of the epidermis (3-5). Salicylic acid reportedly functions by decreasing corneocyte cohesiveness over the entire thickness of the stratum corneum via disruption of desmosomal attachments and denaturing glycoproteins, thus the term “desmolytic” (1,3-5).
The effects of salicylic acid are reported to be more extensive and clinically relevant on skin exhibiting conditions of increased corneocyte cohesiveness (3-5). As a result, dermatologists have used salicylic acid to treat various conditions of hyperkeratosis, including corns, warts, seborrheic dermatitis, psoriasis, and dandruff (2,51). Topical salicylic acid is also a mainstay in home care for acne; this is due to the comedolytic effect of salicylic acid (Fig. 6) (7,52). Aside from OTC formulations, there are products designed for use in dermatologists’ offices including topical peels with concentrations of salicylic acid up to 30%. These products are primarily targeted for the adjunctive treatment of acne, and are also used in photoaging (53-55). Salicylic acid is frequently also used adjunctively in the treatment of psoriasis as a result of its keratolytic effects and its ability to enhance penetration of topical medications (56,57).
The anti-aging benefits of salicylic acid may be limited to the epidermis. Whereas alpha-hydroxy acids have been shown to stimulate biosynthesis of dermal components and increase dermal skin thickness upon topical application, salicylic acid has been shown to decrease dermal skin thicknness (8). Nonetheless, salicylic acid has been used extensively in anti-aging formulations (7), presumably due to its exfoliation effects.
Formulating Factors for Salicylic Acid
Salicylic acid is a somewhat stronger acid with a lower pKa compared to most AHAs due to the electron-withdrawing properties of the benzene ring. As a result, a lower pH should be considered to optimally formulate bioavailable products (Table 1). However, as pH is reduced, and penetration is increased, there is an increased likelihood to cause irritation. The concentration of use of salicylic acid in over-the-counter (OTC) treatments of acne, dandruff, seborrheic dermatitis, psoriasis, and wart formulations is governed by the FDA OTC monographs. These regulatory documents dictate product form, concentration, uses, directions, and warnings. Since these formulations are regulated as OTC drugs, chemical stability of salicylic acid must be proven over the shelf-life of the formulation. Cosmetic formulations that make cosmetic claims, on the other hand, are free to use varying strengths of salicylic acid; however, the upper concentration may be limited by irritation potential.