Given the complexity of the inflammatory process in skin, developing topical products that can effectively resolve the myriad of inflammatory disease states that exist is challenging. By far the most effective and commonly used prescription drugs for treating inflammation are the corticosteroids, particularly the glucocorticoid related steroids. They are very
effective for many forms of eczema, including atopic dermatitis, allergic contact dermatitis, seborrheic dermatitis (in concert with an anti-fungal agent), and have some utility in ameliorating the symptoms of psoriasis. They are not particularly effective, however, in treating acute inflammation, like UVR-induced sunburn, which is not primarily an immune cell driven inflammatory response. Corticosteroids can be used topically or orally. Topical corticosteroids have been classified into groups based on potency. For example, the corticosteroid clobetasol proprionate is ranked as a very potent steroid, while betametasone diproprionate and fluocinolone acetonide can range from potent to moderately potent. OTC topicals containing hydrocortisone are, of course, the least potent (13). Although newer methods are being studied, topical steroid potency is still determined using the MacKenzie vasoconstrictor assay established over 40 years ago. In this assay, a topical steroid is applied to the forearm and the extent and duration of skin blanching due to vasoconstriction assessed by visual examination and rated on a scale of 0 (normal skin, no blanching) to 4 (intense blanching). Although subject to variability, the assay has proved to be a reliable estimate of corticosteroid potency (14).
Given the efficacy of corticosteroids in treating many different types of skin inflammation as well as efficacy in treating autoimmune-based inflammatory diseases such as rheumatoid arthritis, asthma, lupus erythematosus, and allergic rhinitis, considerable research has been directed toward understanding their mechanism of action.
Corticosteroids act on target cells by binding to the glucocorticoid receptor present primarily in the cytosol. This binding “activates” the receptor, resulting in its translocation to the nucleus. The steroid hormone receptor complex then binds, as a homodimer, to DNA regulatory elements along the promoter regions of specific genes. This binding usually results in the up-regulation of gene activity but can also cause transcriptional repression of the target gene (15). The effectiveness of corticosteroids as inhibitors of inflammation stems from the ability of the steroid activated glucocorticoid receptor complex to interfere with the activation of genes regulated, principally, by two transcription factors, NF-kappa B and AP-1 (16,17). These two transcription factors are primarily responsible for the transcriptional activation of a wide variety of pro-inflammatory genes including those for cytokines IL-1, IL-2, IL-3, IL-4, IL-6, IL-11, IL-12, and IL-13, TNF-alpha, and GM-CSF, the chemokine genes IL-8, RANTES, MCP-1, the adhesion molecules ICAM-1, VCAM-1, and E-selectin, the rate-limiting enzyme for PGE-2 production, COX-2, and the matrix- metalloproteinase genes, including MMP-1 (18).
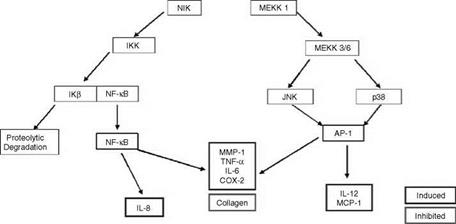
A diagram showing the signaling pathway in cells that leads to the activation of either NF-kappa B or AP-1 and, thus, to the activation of inflammatory genes is shown in Figure 2. Briefly, when a surface receptor on a target cell binds a specific ligand, such as a hormone or cytokine, the receptor is “activated” and this in turn leads to the activation of a signal cascade within the cell. The signaling pathway involves a variety of kinases which are sequentially activated. In the case of the NF-kappa B activation pathway, one of these kinases, IKK, when activated, phosphorylates the protein IkB. This protein, in its unphosphorylated state, binds to NF-kappa B in the cytosol and prevents NF-kappa B from translocating to the nucleus and activating target genes. When IkB is phosphorylated by IKK, it dissociates from NF-kappa B and is degraded. Once freed from the IKB, NF-kappa B can translocate to the nucleus where it binds to the promoter region of specific genes and activates them (19).
As mentioned above, while many inflammatory genes are activated by NF-kappa B, others are regulated by the transcription factor AP-1. This transcription factor is a dimer consisting of either a Jun-Fos heterodimer or a Jun-Jun homodimer. For most cytokine genes, only the Jun-Fos heterodimer functions as a transcriptional activator. As is shown in Figure 2, binding of a ligand such as IL-1 or TNF-alpha to its receptor activates a signaling
|
|
|
cascade that involves sequential activation of a variety of kinases, some of which are members of the MAP kinase family. Either one of two members of this family, JNK (c-Jun N-terminal kinase) or p38 map kinase, when activated by this signaling cascade, phosphorylates, and activates c-Jun and this forms a dimer with the Fos protein to form the functional transcription factor. The Jun-Fos heterodimer will only form if Jun is first phosphoryated by JNK. The Jun-Fos heterodimer forms the AP-1 complex that activates inflammatory target genes (16).
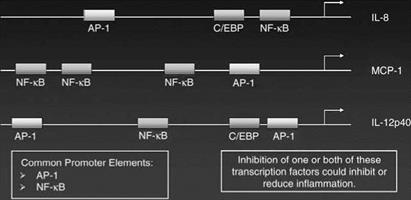
While some genes are regulated only by either NF-kappa B or AP-1 other inflammatory genes have both an NF-kappa B and AP-1 binding site in their promoter regions and, thus can be regulated by either or both transcription factors. Recent data suggests that the control of gene activity by either AP-1 or NF-kappa B depends on both the placement of the transcription factor binding site along the promoter region of the gene and on the level of expression of the transcription factor. To some extent the transcription factor binding site that is closest to the start of transcription plays a predominant role in regulating the activity of the gene. Thus, for example, the MCP-1 gene is strongly regulated by the AP-1 site that lies close to the transcription start site even though there are three NF-kappa B binding sites in the promoter of this gene. Examples of the placement of NF-kappa B and AP-1 transcription factor binding sites in the promoter regions of some inflammatory genes are shown in Figure 3.
As mentioned above, the anti-inflammatory activity of corticosteroids comes from their ability to repress either the activation or activity of the NF-kappa B and AP-1 transcription factors thereby suppressing transcription of genes coding for inflammatory mediators. In the case of NF-kappa B, the actual mechanism of action of the glucocorticoid receptor complex in repressing inflammatory genes activated by this transcription factor is not well understood, but evidence suggests a couple of likely possibilities. One mechanism
Transcriptional Regulation of
Inflammatory Mediators
|
Figure 3 Diagram of transcriptional elements that regulate the activation of inflammatory genes. |
involves the steroid activated glucocorticoid receptor up-regulation of the gene coding for IKB. This produces a cellular excess of this protein which then complexes to and inactivates NF-kappa B, preventing its translocation to the nucleus.
Other data suggests that the glucocorticoid receptor does not block the translocation of NF-kappa B but rather inhibits either binding of the transcription factor to its regulatory site in the promoter region of target genes or alternatively interferes with NF-kappa B’s ability to activate the target gene after binding the promoter region (19-22). Regardless of which specific mechanism is correct, the end result of corticosteroid activation of the glucocorticoid receptor is the repression of NF-kappa B activity and a down-regulation of inflammatory gene activity. In regard to the suppression of the AP-1 stimulation of genes, recent evidence suggests that glucocorticoids block AP-1 phosphorylation and activation by two mechanisms. First, glucocorticoids can suppress AP-1 activity by physically interacting with the Jun component of the dimer, thereby blocking its binding to fos and preventing the formation of an active complex. Secondly, recent studies show that glucocorticoids stimulate the transcription of the MAPK phosphatase-1 gene thereby increasing its abundance in the cell and blocking the phosphorylation of Jun (16).
While the glucocorticoids have been shown to be extremely effective in suppressing the activation of pro-inflammatory genes because of their ability to block NF-kappa B and AP-1 functioning, steroids produce a variety of undesirable side effects. First, due to their potent inhibition of genes involved in an immune cell driven inflammatory response, they have an overall immune suppressive effect. Prolonged use of glucocorticoids leads to a reduction in B – and T-lymphocyte populations, and a reduced ability to fight skin infections. Further, steroids adversely affect the ability of dermal fibroblasts to synthesize collagen and at high doses they reduce the proliferation rate of these cells. Consequently, long-term use of topical steroids can lead to skin thinning and a decrease in the dermal matrix. Other potential negative side effects caused by prolonged use of steroids include
altered carbohydrate metabolism, suppression of the hypothalamic-pituitary-adrenal axis, increased osteoporosis, and increased risk of developing cataracts.
Due to the undesirable side effects which limits the length of time steroids can be used to treat inflammatory diseases, non-steroidal topical therapeutics have been developed to treat inflammation. One group of drugs, the non-steroidal anti-inflammatory drugs (NSAIDs) have been used for many years as oral drugs to control inflammatory responses.