A newer type of NSAIDS is represented by the immunomodulators. Two antiinflammatory drugs that have received FDA approval for topical use are the immunomodulators, Tacrolimus and the related drug Pimecrolimus. These drugs, along with cyclosporine, which exerts its effects through the same mechanism of action, had their origin as immunosuppressive agents used to prevent organ rejection after transplant surgery (41). Although cyclosporine has been used fairly successfully for years as an oral therapeutic for psoriasis, attempts to show that a topical formulation of it is efficacious for this disease have been unsuccessful. Both Pimecrolimus and Tacrolimus have been
approved for topical use in treating atopic dermatitis, but not for psoriasis. However, clinical studies show that systemically delivered Tacrolimus, like cyclosporine, is an effective therapeutic for psoriasis. As is the case with the glucocorticoids, the immunomodulators inhibit the production of inflammatory mediators but unlike the corticosteroids, both Tacrolimus and Pimecrolimus are more cell specific in that they target primarily mast cells and T-lymphocytes. The drugs have fewer inhibitory effects on Langerhans cells/DC, fibroblasts, and keratinocytes (42). Thus, the skin thinning complications seen with topical corticosteroids are eliminated (43,44).
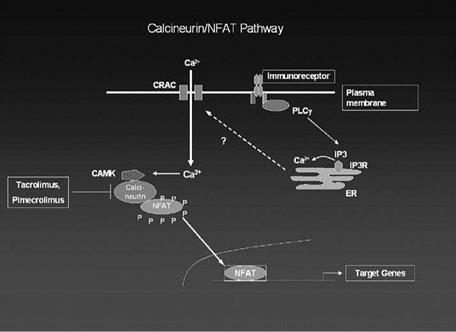
Tacrolimus, Pimecrolimus, and cyclosporine all repress inflammatory genes in target cells through a common mechanism that involves the repression of activity of a ubiquitous calcium-activated phosphatase, calcineurin, that is involved in the activation of specific inflammatory genes (45). When specific receptors on T-cells bind to an antigen, this binding activates the receptor causing an increase in intracellular calcium. The increased calcium causes the activation of calmodulin which then binds to the calcium-dependent enzyme calcineurin and activates it. The activated calcineurin enzyme is a phosphatase, which can dephosphorylate the cytosolic subunit of a transcription factor, nuclear factor of activated T-cells, cytosol (NFATc). The dephosphorylation of the cytosolic NFAT subunit allows it to translocate to the nucleus where it forms a complex with the nuclear subunit of NFAT (NFATn) whose synthesis was induced by the signaling cascade initiated by the antigen binding to the T-cell surface receptor. Once the NFAT dimer has formed in the nucleus, it can bind to the promoter region of several inflammatory genes including those for IL-2, IL-3, IL-4, and TNF-alpha (46,47). A diagrammatic representation of calcineurin activation is shown in Figure 4.
When the drugs Tacrolimus, pimecrolimus, or cyclosporine enter the cell they bind to a cytosol protein, either FKBP for Tacrolimus or Pimecrolimus or Cyclophilin for cyclosporine. Once formed, this complex is able to bind to and inactivate calcineurin.
|
Figure 4 Diagram of calcineurin and NFAT activation. |
The now inactive calcineurin can no longer dephosphorylate NFATc, which results in the transcription factor remaining unactivated and in the cytosol. Thus, the NFATn protein in the nucleus has no binding partner and cannot bind to and activate inflammatory genes (46). One of the genes in T-cells that is inhibited by Tacrolimus is the IL-2 gene, which is necessary for full T-cell activation. Thus, in the presence of these immunomodulators, T-lymphocytes do not differentiate in response to antigen stmulation. In addition to their inhibitory effect on inflammatory gene regulation, these immunomodulators inhibit the degranulation of mast cells, a property which may help explain their efficacy in treating some of the symptoms of atopic dermatitis.
While Tacrolimus and other calcineurin inhibitors are much more specific than corticosteroids in terms of the types of cells they act on, they still inhibit a wide variety of inflammatory genes by inactivating calcineurin and blocking NFAT activation. Another class of immunomodulators, called biologic response modifiers (BRM) or simply “biologics” because they are made from living organisms, have been developed over the past five years (48-50). These are essentially “designer” drugs because they target a specific event or mediator involved in inflammation. Anti-inflammatory drugs in this category include the TNF-alpha inhibitors, Enbrel (etanercept), Remicade (infliximab), and Humira (adalimumab) (51-53). Of these Enbrel has received FDA approval for psorasiatic arthritis and more recently for severe psoriasis. Remicade and Humira have been approved for arthritis and approval for psoriasis is pending. Enbrel is a fusion protein containing the extracellular TNF-alpha binding region of the TNF-alpha receptor. It is injected twice a week by the patient at home. Remicade is a humanized monoclonal antibody to TNF-alpha and is injected intravenously. A second and third dose at two weeks and six weeks after initial dosing is recommended for arthritis (54). Humira is another anti-TNF monoclonal antibody designed to bind TNF-alpha, thereby preventing its attachment to and activation of target cells. Humira is injected every other week by the patient at home.
In addition to the TNF-alpha blockers other BRM drugs that suppress immune responses through different mechanisms have been approved for use in treating various forms of inflammation (55). Two of these, Raptiva (Efalizumab) and Amevive (Alefacept) have been approved as injectables for treating arthritis. Amevive, the first FDA approved drug for psoriasis, is a dimeric fusion protein containing the CD-2 binding site of the leukocyte antigen, LFA-3. When injected (once a week) Amevive binds to the CD2 binding site on T-lymphocytes thereby preventing binding between the LFA-3 antigen present on APC and the CD2 binding site on T-lymphocytes. Thus, the lymphocytes are not activated by antigen presentation. Another “humanized,” “biologic” therapeutic which is injected weekly is the monoclonal antibody, called Raptiva, which binds to CD11a, which is part of the LFA-1 protein expressed on leukocytes. By occupying this binding site Raptiva prevents the leukocytes from binding an adhesion molecule, ICAM, which is present on endothelial cells. By preventing the adhesion of T-lymphocytes to the blood vessel wall, Raptiva prevents the activation of T-lymphocytes as well as their movement into the skin, thereby reducing the level of T-cell mediated inflammation. CD11a is also expressed on the surface of B-lymphocytes, monocytes, neutrophils, natural killer cells, and other leukocytes. Thus, Raptiva has the potential to down-regulate responses by other immune cells further reducing inflammatory responses (52).
These new protein-based “biologic” immunomodulators, although effective and useful for treating various dermatological conditions, are, however, not without side effects. Because of their potent immunosuppressive effects, particularly on T-lymphocytes, the risk of infection among patients taking these medications is elevated (56-58). Enbrel, for example, has been found, in post-marketing use, to cause serious infections, sepsis, and even fatalities in patients predisposed to infections, and this warning is now included with
the drug information. Further, as is the case with all of protein-based biological response modifier drugs, none are capable of being delivered topically because of their size.
Given the myriad of immune driven events which occur in skin in response to exposure to antigens or other external stimuli, it is easy to see why immunomodulators and biologics are effective in treating inflammatory diseases such as atopic dermatitis and psoriasis. In the case of Tacrolimus and Pimecrolimus, by blocking the calcineurin pathway, these drugs can suppress the activity of the TNF-alpha and IL-2 genes in T-lymphocytes, thus preventing the activation of these lymphocytes as well as preventing their binding to adhesion proteins along the endothelium. Further, recent studies have shown that the calcineruin/NFAT pathway is active in epidermal keratinocytes and inhibited by either cyclosporine or Tacrolimus (47). Since keratinocytes produce a variety of inflammatory mediators such as IL-1 and TNF-alpha which, in turn, exert effects on a number of cells including fibroblasts (up-regulate PGE-2, cytokines), mast cells (degranulation) and endothelial cells (increased expression of adhesion moledule, ICAM, and VCAM, an inhibitory effect on IL-1 and TNF-alpha production would slow the production of inflammatory mediators and suppress the movement of immune cells into the skin. Similarly, the BRMs can be expected to be effective treatments for atopic dermatitis, and psoriasis based on their designed function of either blocking TNF-alpha action or T-lymphocyte activation. However, as mentioned these drugs can only be used by injection and not applied topically.