Anagen is followed by a phase of hair follicle involution, catagen. Catagen was first characterised in detail by Kligman [69] and Straile [70]. At the beginning of catagen, proliferation and differentiation of hair matrix keratinocytes reduces dramatically, the pigment-producing activity of melanocytes ceases, and hair shaft production is completed. During catagen, the follicle compartments involved in hair production are reduced to sizes that allow them to regenerate in the next hair cycle after receiving the appropriate stimulation. The hair follicle shortens in length by up to 70%. Although catagen is often considered a regressive event, it is an exquisitely orchestrated, energy-requiring remodelling process, whose progression assures renewal of a further generation of the hair follicle. Morphologically and functionally, catagen is divided into eight sub-stages [59]. During catagen, a specialised structure, the club hair is formed. The keratinised brush-like structure at the base of the club hair is surrounded by epithelial cells of the outer root sheath and anchors the hair in the telogen follicle. During catagen, the dermal papilla is transformed into a cluster of quiescent cells that are closely adjacent to the regressing hair follicle epithelium and travel from the subcutis to the dermis/subcutis border to maintain contact with the distal portion of the hair follicle epithelium including the secondary hair germ and bulge. Catagen is characterised by several simultaneously occurring and tightly coordinated cellular programs. The most important characteristic feature is a well-coordinated
apoptosis occurring in the proximal part of the hair follicle. Apoptosis is regulated differently in each follicle compartment and distinct cell populations show different abilities to undergo apoptosis [55]. The majority of the follicular epithelial cells and melanocytes are very susceptible to apoptosis, while dermal papilla fibroblasts and the populations of keratinocytes and melanocytes selected for survival display a high resistance [71,72]. The physiological involution of the hair follicle may be triggered by the withdrawal of dermal papilla-derived growth factors that maintain cell proliferation and differentiation in the anagen hair follicle, and by a variety of stimuli, including signalling via death receptors (Fig. 1.4).
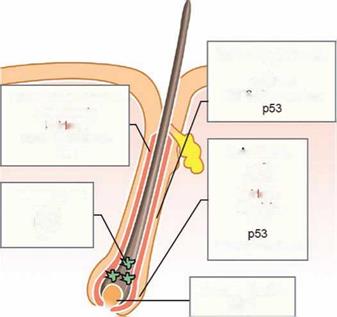 One of the candidate molecules mediating apoptosis in hair matrix keratinocytes after growth factor withdrawal is p53. Mice lacking p53 showed significantly retarded catagen progression, compared with control mice confirming a pro-apoptotic role for p53 in the hair follicle [26]. The delicate proliferation-apoptosis balance, essential for follicle cyclic behaviour, can also be controlled by survivin [73]. Survivin, a member of the apoptosis inhibitor protein family, is implicated in the control of cell proliferation as well as the inhibition of apoptosis [74]. Survivin, expressed in the proliferating keratinocytes of the anagen hair matrix and outer root sheath, disappears with the progression of catagen [73]. Before or during catagen, outer root sheath keratinocytes produce several important catagen-promoting secreted molecules: fibroblast growth factor-5 short isoform, neurotrophins, transforming growth factor-в 1/2 (TGF-P1/2), IGF binding protein 3, and thrombospondin-1 [75-78]. Several important growth factors were discovered as modulators of catagen development
One of the candidate molecules mediating apoptosis in hair matrix keratinocytes after growth factor withdrawal is p53. Mice lacking p53 showed significantly retarded catagen progression, compared with control mice confirming a pro-apoptotic role for p53 in the hair follicle [26]. The delicate proliferation-apoptosis balance, essential for follicle cyclic behaviour, can also be controlled by survivin [73]. Survivin, a member of the apoptosis inhibitor protein family, is implicated in the control of cell proliferation as well as the inhibition of apoptosis [74]. Survivin, expressed in the proliferating keratinocytes of the anagen hair matrix and outer root sheath, disappears with the progression of catagen [73]. Before or during catagen, outer root sheath keratinocytes produce several important catagen-promoting secreted molecules: fibroblast growth factor-5 short isoform, neurotrophins, transforming growth factor-в 1/2 (TGF-P1/2), IGF binding protein 3, and thrombospondin-1 [75-78]. Several important growth factors were discovered as modulators of catagen development
Figure 1.4 Molecular mechanisms of apoptosis control in the distinct hair follicle compartments. Scheme demonstrates the expression pattern of anti – and pro-apoptotic molecules (shown in brown and black respectively) in the hair follicle.
by gene knockout studies. The most remarkable phenotype was seen in mice lacking the fibroblast growth factor-5 (Fgf5) gene whose hair was 50% longer than their wild type lit – termates, giving an “angora-like” phenotype [79]. Neurotrophins and TGF-^1 also induce premature catagen onset. Mice overexpressing distinct members of the neurotrophin family (BDNF, NT-3) show premature catagen development in part by stimulation of pro – apoptotic signalling through the p75 kD neurotrophin receptor in the outer root sheath [75]. TGF-^1 knockout mice display delayed catagen onset [76]. Neurotrophins and TGF-^2 also exert catagen-promoting effects on human hair follicles in organ culture [80,81]. Catagen can also be initiated by several other molecules, such as endothelin-1, insulinlike growth factor binding proteins-3/4/5, interleukin-1, vitamin D receptor (reviewed in [82]), prolactin [83,84], endocannabinoids [85], or thrombospondin-1 [78].
