1.7.2.1 Human Hair Follicles Show Paradoxically Different Intrinsic Responses to Androgens
Androgens’ dramatic stimulation of hair growth is seen first in puberty with pubic and axillary hair development in both sexes [16-18]. These changes parallel the rise in plasma androgens, occurring later in boys than girls [146, 147]. Testosterone stimulates beard growth in eunuchs and elderly men [148] and castration inhibits beard growth [49] and male pattern baldness [149], but individuals with complete androgen insufficiency (i. e. without functional androgen receptors) highlight the essential involvement of androgens [150]. As they cannot respond to androgen, these XY individuals develop a female – type phenotype, but without any pubic or axillary hair or any androgenetic alopecia (Fig. 1. 2). Growth hormone is also required for the full androgen response as sexual hair development is inhibited in growth hormone deficiency [151].
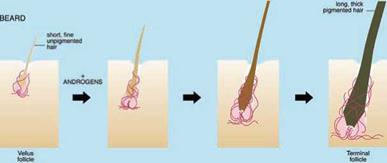
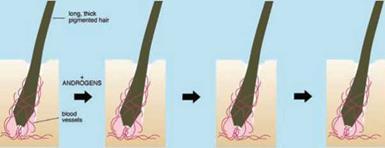
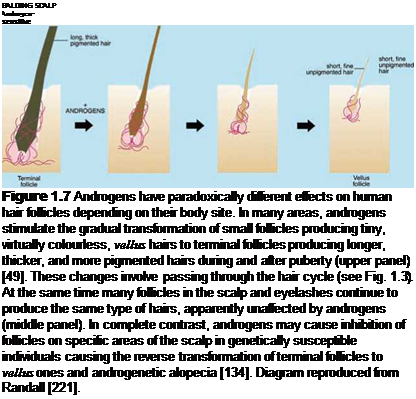
Androgens stimulate tiny vellus follicles producing fine, virtually colourless, almost invisible hairs to transform into larger, deeper follicles forming longer, thicker, more pigmented hairs (Fig. 1.7). Follicles must pass through the hair cycle, regenerating the lower follicle to carry out such changes (Section 1.4). Although androgens stimulate hair growth in many areas, causing greater hair growth on the face, upper pubic diamond, chest, etc. in men [49], they can also have the opposite effect on specific scalp areas, often in the same individual, causing balding [57]. This involves the reverse transformation of large, deep follicles producing long, often heavily pigmented terminal scalp hairs to miniaturised vellus follicles forming tiny, almost invisible hairs (Fig. 1.7).
During puberty, the hairline is usually straight across the top of the forehead. In many men this frontal hairline progressively regresses in two wings and thinning occurs
|
|
|
|
|
NON-BALDING SCALP Androgen independent |
mid-vertex [134]. These areas gradually expand in a precise pattern exposing ‘bare’ scalp [134,152]; the lower sides and back normally retain terminal hair (Fig. 1.2). Androgenetic alopecia is reviewed thoroughly elsewhere [20,153]. Similar hair loss, considered androgen – dependent, can occur in women, but the pattern differs; the frontal hairline is normally retained while generalised thinning progresses on the vertex until it appears bald [154].
In contrast, androgens appear to have no effect on other hairs like the eyelashes (Fig. 1.7). This is an intriguing and unique biological paradox. How does one hormone stimulate an organ, the hair follicle, in many areas, but have no effect in another, while at the same time, cause inhibition in the same organ in another part of the body, often in the same individual?
There are also significant differences between androgen-stimulated follicles. Axillary and lower pubic follicles enlarge in response to female levels of androgens, while other follicles require male levels [146,147]. Follicles also differ in their sensitivity, or speed of response. Facial follicles enlarge first above the mouth (moustache) and on the chin in boys and hirsute women; this spreads gradually over the face and neck [18]. This progression resembles the patterned inhibition during balding [134,152]. Many androgen responses are gradual, with some follicles taking years to show the full response. Beard weight increases dramatically during puberty but continues rising until the mid-thirties, while terminal hairs may only be visible on the chest and ear canal years later [49] and the miniaturisation processes of androgenetic alopecia continue well into old age [134,152]. This delay parallels the late onset of androgen-dependent benign prostatic hypertrophy and prostatic carcinoma [135].
Another demonstration of the intrinsic behaviour of human follicles is the contrast between beard and axillary hair growth. Although both increase rapidly during puberty, beard growth remains heavy, while axillary hair is maximal in the mid-twenties before falling rapidly in both sexes [49].This is another paradox; why do follicles in some areas no longer show their androgenic responses, while in many others they maintain or extend them?
These contrasts are presumably due to differential gene expression within individual follicles, since all follicles are exposed to the same circulating hormones and, from the complete androgen insensitivity syndrome, require the same receptor. [150]. Follicles’ retention of their original androgen response when transplanted, the basis of corrective cosmetic surgery confirms this [156]. Presumably, this genetic programming occurs, in the patterning processes during development. Interestingly, the dermis of the chick’s frontal parietal scalp, which parallels human balding regions, develops from the neural crest, while the occipital-temporal region, our non-balding area, arises from the mesoderm [157 ] . The molecular mechanisms involved in forming different types of follicles during embryogen – esis are unclear, but secreted signalling factors, such as Eda, sonic hedgehog, Wnt, and various growth factor families (e. g. BMPs, nuclear factors), including various homeobox genes, and others such as Hairless and Tabby, plus transmembrane and extracellular matrix molecules are all implicated [158,159].
Human follicles require androgens not only for their initial transformation, but also need them to maintain many of the effects. If men are castrated after puberty neither beard growth nor male pattern balding return to prepubertal levels [22,134] suggesting that some altered gene expression does not require androgens for maintenance or lower levels can maintain some effect. Nevertheless, beard growth increases in the summer [11] (Fig. 1.6), probably in response to increased circulating androgens (Section 1.6), antiandrogen treatment reduces hair growth in hirsutism [160 ] and more selective blockers of androgen action, 5a-reductase inhibitors such as finasteride, can cause regrowth in androgenetic alopecia [161,162]. This suggests that androgens are required to maintain most of the responses, as well as initiating progression.
These intrinsic differences in hair follicle androgen responses have important consequences for anyone wishing to investigate androgen action. It is essential to study follicles which respond appropriately in vivo for the question being addressed. Unfortunately, this means that the most available human material, non-balding scalp, is often inappropriate.
Genetics also appears important in androgen-dependent hair growth. Male pattern baldness [149,163,164] and heavy beard growth [49] run in families, Caucasian men and women generally have greater hair growth than Japanese [49], despite similar testosterone levels [165], and African men exhibit much less baldness [21]. Several genes have been investigated for association with androgenetic alopecia. Interestingly, women with polycystic ovaries and their brothers with early balding exhibit links to one allele of the steroid metabolism gene, CYP17 [166 ] . No association was found with neutral polymorphic markers of genes for testosterone metabolising enzymes 5a-reductase type-1 or -2 in balding [167,168]; however, StuI restriction fragment length polymorphism (RFLP) in exon 1 of the androgen receptor was present in young (98%) and older (92%) balding men, although also in 77% of older controls [169]. Although single triplet repeats of CAG or GAC were unaltered, short/short polymorphic CAG/GGC haplotypes were significantly higher in balding subjects. Interestingly, Spanish girls with precocious puberty (i. e. before 8 years) showed smaller numbers of CAG repeats [170] and shorter triplet repeat lengths are associated with another androgen-dependent condition, prostate cancer [171]. Whether this has functional significance like increased androgen sensitivity or simply reflects linkage disequilibrium with a causative mutation is unclear. However, increased sensitivity is not supported by the similarity of steroid binding capability between androgen receptors from balding and non-balding follicle dermal papilla cells [172].