Specific effects of androgens on hair follicle cells. Androgens must alter many aspects of follicular cell activity to cause these changes in follicle and hair type. They must alter the ability of epithelial matrix cells to divide, determine whether they should differentiate into medulla (found in some large hairs), and regulate the pigment produced and/or transferred by follicular melanocytes. They must also alter dermal papilla size which has a constant relationship with the hair and follicle size [173 ] 174], and ensure the dermal sheath surrounding follicles expands to accommodate larger follicles. These responses are also quite complex; for example, altering hair length could involve changing cell division rate, that is, hair growth rate, and/or the actual growing period, anagen. Anagen length seems the most important. Thigh hair is three times longer in young men than women, but grows only slightly faster for a much longer period [175]. Androgens do cause such alterations as antiandrogen treatment reduces hair diameter, growth rate, length, pigmentation, and medulla – tion in hirsute women [176], while blocking 5a-reductase activity increases many of these aspects in alopecia [161]. This raises the question: are androgens acting on each target cell individually or operating through one coordinating system with indirect effects on other cell types?
General mechanism of action of androgens. Androgens, like other steroid hormones, diffuse through cell membranes to act on target cells by binding to specific intracellular receptors. These hormone-receptor complexes undergo conformational changes exposing DNA binding sites and bind to specific hormone response elements (HRE) in the DNA, often in combination with accessory (coactivating) proteins, promoting expression of specific, hormone-regulated genes [177]. Androgen action is more complex than other steroids. Testosterone, the main male circulating androgen, binds receptors in some tissues (e. g. skeletal muscle). However, in others, including secondary sexual tissues like the prostate, testosterone is metabolised intracellularly by 5a-reductase enzymes to 5a-dihydrotestosterone, a more potent androgen, which binds more strongly to the androgen receptor to activate gene expression [178].
Androgen-dependent follicles require androgen receptors to respond as highlighted by the absence of adult body hair in complete androgen insensitivity (Fig. 1.2) [150], but the need for 5a-reductase varies with body region. Men with 5a-reductase type-2 deficiency only produce female patterns of pubic and axillary hair growth, although their body shapes become masculinised [179] (Fig. 1.2). Therefore, 5a-dihydrotestosterone appears necessary for follicles characteristic of men, including beard, chest, and upper pubic diamond, while testosterone itself can stimulate the axilla and lower pubic triangle follicles also found in women. Since androgenetic alopecia is not seen in 5a-reductase type-2 deficient men and the 5a-reductase type-2 inhibitor, finasteride, can restore hair growth [85,86], 5a-reductase type-2 also seems important for androgen-dependent balding.
Why some follicles need 5a-dihydrotestosterone and others testosterone to stimulate the same types of cell biological changes that lead to larger hairs is unclear; presumably, the cells use different intracellular coactivating proteins to act with the receptor.
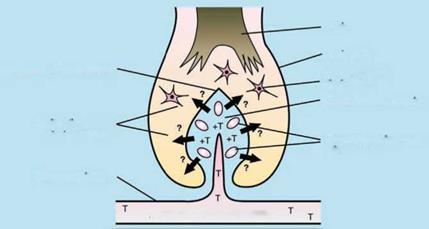
Current model for androgen action in hair follicles. Hair follicle growth is complex but rarely abnormal, indicating a highly controlled system. This suggests that androgen action is coordinated through one part of the follicle. The current hypothesis, proposed in 1990 by Randall et al. [180], focuses on the dermal papilla with androgens acting directly on dermal papilla cells where they bind to androgen receptors and then initiate the altered gene expression of regulatory factors which influence other target cells (Fig. 1.8). These factors could be soluble paracrine factors and/or extracellular matrix factors; extracellular matrix forms much of the papilla volume, and dermal papilla size corresponds to hair and follicle size [173, 174]. In this model the dermal papilla is the primary direct target, while other cells such as keratinocytes and melanocytes are indirect targets.
This hypothesis evolved from several concepts reviewed elsewhere [3 , 180] including dermal papilla determination of the type of hair produced [89]; adult follicle cycles partially recapitulating their embryogenic development; strong parallels in androgen dependency and age-related changes between hair follicles and the prostate; and androgens acting on embryonic prostate epithelium through the mesenchyme [155]. There is now strong experimental support for this model. Androgen receptors are found in the dermal papilla [126,181] and in cultured dermal papilla cells derived from androgen-sensitive follicles including beard [135], balding scalp [172], and deer manes [182]. Cells from androgen – sensitive sites contain higher levels of specific, saturable androgen receptors than androgen-insensitive non-balding scalp in vitro [135,172,183]. Importantly, beard, but not pubic or non-balding scalp cultured dermal papilla cells metabolise testosterone to 5a – dihydrotestosterone in vitro [184-186] reflecting hair growth in 5a-reductase deficiency;
 T CIRCULATING ANDROGENS
T CIRCULATING ANDROGENS
Figure 1.8 The current model for androgen action in the hair follicle. In this model androgens from the blood enter the hair follicle via the dermal papilla ‘s blood supply. They are bound by androgen receptors in the dermal papilla cells causing changes in their production of regulatory paracrine factors; these then alter the activity of dermal papilla cells, follicular keratinocytes, melanocytes, etc. T = testosterone; ? = unknown paracrine factors. Reproduced from Randall [221].
5a-reductase type-2 gene expression also supports this [183]. These results led to wide acceptance of this hypothesis.
However, some recent observations suggest minor modifications. The dermal sheath, which isolates the follicle from the dermis, now seems to have other important roles as well, as it can form a new dermal papilla and stimulate follicle development [187]. Cultured dermal sheath cells from beard follicles contain similar levels of androgen receptors to dermal papilla cells (personal observations) and balding dermal sheath and dermal papilla express mRNA for 5a-reductase type-2 [188]. This indicates that the dermal sheath can respond directly to androgens without the dermal papilla acting as an intermediary. The sheath may be a reserve to replace a lost dermal papilla’s key roles because of hair’s essential role for mammalian survival and/or dermal sheath cells may respond directly to androgens to facilitate alterations in sheath, or even dermal papilla, size in forming a differently sized follicle.
Recently, a very specialised keratin, hHa7, was found in the medulla of hairs from beard, pubis, and axilla [189]. The medulla is formed by central hair cells which develop large air-filled spaces. Beard medulla cells showed coexpression of keratin hHa7 and the androgen receptor. Since the hHa7 gene promoter also contained sequences with high homology to the androgen response element (ARE), keratin hHa7 expression may be androgen – regulated. However, no stimulation occurred when the promoter was transfected into prostate cells and keratin hHa7 with the same promoter is also expressed in androgen-insensitive body hairs of chimpanzees [190] making the significance unclear. Nevertheless, the current model needs modification to include possible specific, direct action of androgens on lower dermal sheath and medulla cells.
The alteration of signalling molecules in the hair follicle by androgens. The final part of the mechanism of androgen action involves the alteration of paracrine signalling factors produced by dermal papilla cells. There is great interest in paracrine signalling in developing and cycling follicles, aiming to understand hair follicles as dynamic organs (see Sections 1.2 and 1.3) [90,190]. Unfortunately, there are few practical animal models for studying androgen effects [191] because of the special effects of androgens on human follicles. Fortunately, cultured dermal papilla cells from follicles with different sensitivities to androgens offer a useful model in which to study androgen effects due to the dermal papilla’s central role, their abilities to be grown from small skin samples, to stimulate hair growth in vivo at low passage numbers [89,90], and to retain characteristics in vitro which reflect their androgen responses in vivo [191] (discussed earlier). They secrete both extracellular matrix [192] and soluble, proteinaceous factors which stimulate growth in other dermal papilla cells [180,193], outer root sheath cells [194,195], and transformed epidermal keratinocytes [196]. Soluble factors from human cells can cross species affecting rodent cell growth in vitro and in vivo [197], paralleling the ability of human dermal papillae to induce hair growth in vivo in athymic mice [198].
Importantly, physiological levels of testosterone in vitro increase the ability of beard cells to promote increased growth of other beard dermal papilla cells [193], outer root sheath cells [195], and keratinocytes [196] in line with the hypothesis. Interestingly, testosterone had no effect on non-balding scalp cells and only beard cells responded to the soluble factors produced [193], suggesting they have different receptors to non-balding scalp cells. This implies that an autocrine mechanism is involved in androgen-stimulated beard cell growth; androgen-mediated changes do involve alterations in dermal papilla cell numbers as well as the amount of extracellular matrix [174]. A need to modify the autocrine production of growth factors could contribute to the slow androgenic response, which often takes many years to reach full effect [22,134]. In contrast to the beard cell stimulation, testosterone decreased the mitogenic capacity of androgenetic alopecia dermal papilla cells from both men [196] and stump-tailed macaques [199]. All these results support the dermal papilla based model and demonstrate that the paradoxical androgen effects observed in vivo are reflected in vitro, strengthening the use of cultured dermal papilla cells as a model system for studying androgen action in vitro.
The main priority now is to identify the factors that androgens alter. So far, only IGF-1 is identified as secreted by beard cells under androgens in vitro [181]. IGF-1 is a potent mitogen which maintains anagen in cultured human follicles [109,200] and abnormal hair growth occurs in the IGF-I receptor deficient mouse [201] supporting its importance. Beard cells also secrete more SCF than non-balding scalp cells, although this is unaltered by androgens in vitro [202]. Since SCF plays important roles in epidermal [203] and hair pigmentation development [204], the dermal papilla probably provides local SCF for follicular melanocytes [202]. Androgens in vivo presumably increase scf expression by facial dermal papilla cells to cause hair darkening when boys’ vellus hairs transform to adult beard. Recently DNA microarray methods also revealed that three genes, sfrp-2, mnl, and atplfil, were expressed at significantly higher levels in beard than normal scalp cells, but no changes were detected due to androgen in vitro [205].
Although androgenetic alopecia dermal papilla cells are even more difficult to culture than normal follicles [206], androgens inhibit their expression of protease nexin-1, a potent inhibitor of serine proteases, which regulate cellular growth and differentiation in many tissues [207]. Androgens also stimulate their production of TGF-в and TGF-^2 [208,209]. TGF-в is a strong candidate for an inhibitor of keratinocyte activity in alopecia because it inhibits human follicle growth in vitro promoting catagen-like changes in human beings [111,210] and mice [211]; a probable TGF-^1 suppressor delays catagen in mice [212] and follicular keratinocytes have receptors for TGF-fl [213]. However, in a limited DNA macroarray analysis TGF-^2 and TNF-a were actually slightly reduced in balding cells [214]. Balding scalp-cell conditioned media also inhibits human and rodent dermal papilla cell growth in vitro and delays mouse hair growth in vivo suggesting active secretion of inhibitory factors [197]. This is unlikely to involve TGF-в which is associated with the transition from anagen to catagen [210,211] and whose receptors are only detected on keratinocytes [213]. Thus, studying dermal papilla cells implicates several factors already: IGF-1 in enlargement, SCF in increased pigmentation, and nexin-1 and TGF-в in miniaturisation. Alterations in several factors are probably necessary to precisely control the major cell biological rearrangements required when follicles change size. Further research into such factors should help clarify the complex follicular cell interactions and the pathogenesis of androgen – dependent disorders.
