The skin plays many roles ranging from barrier function to highly complex biochemical and photobiochemical processes. If we follow the definition above then cosmeceuticals are inherently not simply cosmetics to beautify the appearance of the skin.
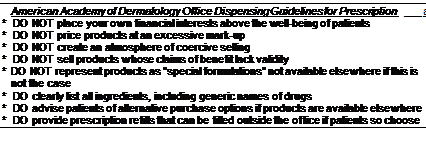 |
Figure 1 American Academy of Dermatology Office Dispensing Guidelines for Prescription and Non-prescription Products. Source: American Academy of Dermatology, 2003.
Cosmeceuticals then affect either the structure or the function (or both) of the skin. Unlike drugs, cosmeceuticals typically are very safe and have few significant serious adverse events. However, like drugs, these active agents can impact many diverse functions of the skin and we do not fully comprehend the implications of these actions in many cases.
For example, take botanical-based actives in cosmeceuticals. These plant-derived substances have the potential for contact dermatitis like reactions similar to poison ivy dermatitis. Irritant reactions are also possible as are phytophotodermatoses. Typically products are selected which do not pose these concerns and also the concentrations used in the formulations are below the threshold of reaction (12).
There are also issues of bioequivalency. Most physicians recall the use of digitalis in past for cardiac treatments—before standardized digoxin became available. An example with cosmeceuticals is that the polyphenol content of a particular botanical may vary from brand to brand; even though the percentage concentration of that active seems equal among brands, the difference in polyphenol content may make one product less efficacious than the other brand, which has a higher content of polyphenols (13).
Another example of an issue is with one of the very popular alpha-hydroxy acid (AHA) actives, glycolic acid, where the pH and pKa values impact the clinical effects and side effects. For these products, simply comparing the percentage of glycolic acid did not provide the physician, esthetician, or consumer with an accurate assessment of the effects on the skin. In fact, a lower percent glycolic acid product could potentially be more irritating than a higher percentage glycolic acid product depending on the pH (14,15).
An issue infrequently discussed is that of pesticide residues or other contaminants for botanicals. So bioequivalency and bioavailability and purity are all issues for these types of active ingredients. Also, while we are thinking in the “drug” pattern, the “dose” is important. So the percentage of actual active ingredient also determines to some extent the effects of cosmeceuticals compared to Rx drugs. Also, physician-dispensed products often have a higher “dose” or concentration of actives than the OTC products.
Other factors are synergistic reactions and stability. Many cosmeceutical formulations have complex mixtures of actives the interactions of which are not all well defined. Some antioxidants are not that stable and others may be unstable after they are opened. Novel new “airless” pump delivery systems or mixing as pumped onto skin from applicator provide ways to combat these problems.
Data on the relative potency is often lacking on active ingredients within the same category. Antioxidants are a good example, and this data has only recently begun to be published. Much of the data about the drug-like effects on the skin’s structure and function are considered proprietary and not available for physicians or the consumer to review. Additionally many products are sold widely with minimal scientific or clinical data whatsoever. Thus, while cosmeceuticals increasingly affect the skin’s structure and function like drugs, the data that is traditionally available for drug evaluation is often incomplete or nonexistent. The great safety of most cosmeceutical actives is one of the mitigating factors in this scenario. We will see antioxidants grow dramatically in their role with the cosmeceutical armamentarium to protect and also in some cases repair environmental damage and aging in general.
In summary, cosmeceuticals may have profound effects on the structure and/or function of the skin—or they may have little or no effect and behave like cosmetics. A comprehensive discussion of this is beyond the scope of this section, but the use of cosmeceuticals to improve the appearance and health of the skin is a fascinating area of science and one which we will see explored and mapped in the years ahead.
